Welcome to the fascinating world of enzymes and their active sites! Have you ever wondered how these tiny biological catalysts work their magic to speed up reactions in your body? In this blog post, we will dive into everything you need to know about active sites, from how they increase reaction speeds to their crucial role in breaking down toxins and aiding muscle function. So, grab a cup of tea and let’s unravel the mysteries of enzyme activity together!
What is the active site of an enzyme?
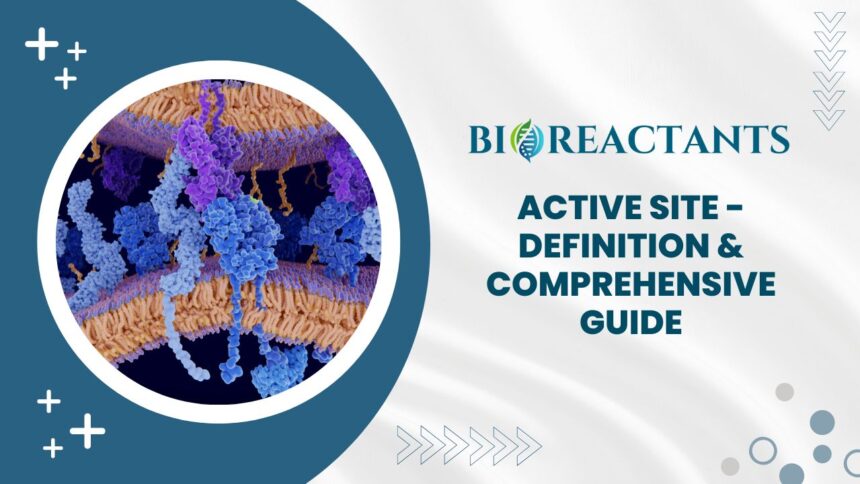
The active site of an enzyme is like a keyhole that perfectly fits its specific substrate, the molecule it acts upon. It’s where all the action happens in enzymatic reactions. Picture a lock and key mechanism – only when the right key (substrate) enters the precise lock (active site) can the door (reaction) open smoothly.
This pocket within the enzyme’s structure contains amino acids that interact with the substrate, creating an environment conducive to chemical reactions. Think of it as a miniature workspace where molecules are transformed into products at lightning speed.
Enzymes are incredibly selective, recognizing their target substrates with precision due to complementary shapes and charges between the active site and substrate. This specificity ensures that enzymes catalyze only specific reactions while maintaining efficiency.
Without these crucial active sites, enzymes would be powerless to carry out their essential functions in our bodies, from digestion to energy production. They truly are molecular marvels designed for biochemical perfection!
How do enzymes increase reaction speed?
Enzymes are like the superheroes of the biochemical world, swooping in to save the day by speeding up chemical reactions. Picture a bustling city street – enzymes are the traffic lights directing cars smoothly through intersections, making everything flow efficiently.
The secret behind their super-speed powers lies in their ability to lower the activation energy needed for a reaction to occur. Enzymes act as catalysts, reducing the energy required for molecules to collide and transform into products.
Think of enzymes as matchmakers bringing together substrates (reactants) in just the right orientation for them to react quickly and effectively. By providing a suitable environment at their active sites, enzymes create optimal conditions for reactions to take place rapidly.
In essence, enzymes work by facilitating collisions between molecules and holding them in positions that promote bonding and chemistry magic. They’re like precision engineers fine-tuning every step of a process with remarkable efficiency and accuracy.
What determines active site specificity?
The active site specificity of an enzyme is determined by its unique three-dimensional structure. This structure allows the enzyme to recognize and bind to specific substrates like a lock and key fitting perfectly together. The amino acid residues present in the active site play a crucial role in determining this specificity, creating a microenvironment that is ideal for catalyzing specific reactions.
Additionally, interactions between the enzyme’s active site and the substrate govern how effectively the reaction can proceed. Subtle changes in either the enzyme or substrate can impact this interaction, influencing the efficiency of enzymatic activity.
Enzymes have evolved over time to exhibit high levels of specificity towards certain substrates, enabling them to carry out their biological functions with precision. Understanding what determines active site specificity sheds light on how enzymes function with remarkable efficiency in living organisms.
What are the two theories of active site binding?
When it comes to active site binding, two main theories are widely discussed in the realm of enzymology. The first theory is the lock and key model, which suggests that enzymes and substrates fit together perfectly like a key fits into a lock. This model implies specificity in enzyme-substrate interactions, where only certain substrates can bind to specific enzymes.
On the other hand, the induced fit model proposes a more dynamic approach. It suggests that enzymes undergo conformational changes upon substrate binding, leading to a better fit between the enzyme and substrate molecules. This flexible nature allows enzymes to adapt their active sites to accommodate different substrates.
While both theories offer valuable insights into how enzymes interact with substrates, the induced fit model is more widely accepted due to its ability to explain various experimental observations in enzymatic reactions.
Which model is more widely accepted?
When it comes to the two theories of active site binding, scientists have debated for years on which model is more widely accepted. The lock and key model suggests that the enzyme’s active site perfectly fits the substrate like a key in a lock. On the other hand, the induced fit model proposes that the active site changes its shape slightly to accommodate the substrate.
While both models have their merits, recent studies lean towards favoring the induced fit model due to its flexibility and ability to explain a wider range of enzyme-substrate interactions. This dynamic interaction between enzymes and substrates allows for better understanding of how enzymes catalyze reactions with high specificity and efficiency.
By acknowledging the adaptability of enzymes in conforming to substrates, researchers continue to unravel the complexities of enzymatic processes at molecular levels.
Give examples of enzyme-catalyzed reactions.
Enzymes play a crucial role in catalyzing a wide range of reactions in living organisms. For example, amylase is an enzyme that breaks down starch into sugar molecules like glucose in the digestive system. This process helps in the digestion of carbohydrates and provides energy to the body.
Another example is catalase, which helps convert hydrogen peroxide into water and oxygen. This reaction protects cells from oxidative damage by neutralizing harmful compounds.
Furthermore, lipase is an enzyme responsible for breaking down fats or lipids into fatty acids and glycerol. Without this enzyme, our bodies would struggle to absorb essential nutrients from fat-rich foods.
Enzyme-catalyzed reactions are essential for maintaining various biological processes and keeping our bodies functioning optimally.
Where is maltase found, and what does it break down?
Maltase is an enzyme that plays a crucial role in breaking down maltose, a disaccharide composed of two glucose molecules linked together. This enzyme is primarily found in the small intestine, where it aids in the digestion process by catalyzing the hydrolysis of maltose into two individual glucose molecules. By doing so, maltase enables the body to absorb these simpler sugar units more efficiently for energy production and other essential functions.
In addition to its presence in the small intestine, maltase can also be found in certain cells throughout the body that require glucose as a primary energy source. Its ability to break down complex sugars like maltose into readily usable forms such as glucose underscores its significance in maintaining proper metabolic processes and overall health.
What are the catalytic groups in an active site?
The catalytic groups in an active site play a crucial role in enzyme function. These groups are responsible for facilitating the chemical reactions that enzymes catalyze. The most common catalytic groups include amino acids such as histidine, serine, and cysteine.
These specific amino acids have unique properties that allow them to participate in the enzyme’s mechanism of action. For example, histidine can act as a proton donor or acceptor, while serine and cysteine can form covalent bonds with substrates during the reaction process.
By leveraging these catalytic groups, enzymes are able to speed up biochemical reactions by lowering the activation energy required for the reaction to occur. This accelerates vital processes within living organisms and ensures metabolic activities proceed efficiently.
Understanding how these catalytic groups operate within an enzyme’s active site provides valuable insights into how biological molecules interact and transform in intricate ways. By unraveling this molecular dance, scientists continue to uncover new avenues for drug development and biotechnological advancements.
What is the role of substrate binding sites?
Enzymes have specific pockets called substrate binding sites where the substrate molecules bind. These binding sites are like puzzle pieces that perfectly fit together with the substrates, ensuring a precise and efficient reaction. The shape and chemical properties of the active site determine which substrates can bind to it.
When a substrate fits into the enzyme’s active site, it forms temporary bonds with the enzyme, allowing for chemical reactions to occur at a faster rate. This interaction stabilizes the transition state of the reaction, lowering its activation energy.
The substrate binding sites play a crucial role in catalyzing biochemical reactions by bringing together reactants in an optimal orientation for bonding to occur. This process enhances reaction specificity and efficiency, enabling enzymes to perform their functions effectively within biological systems.
Why are enzymes essential for life?
Enzymes are the unsung heroes of our bodies, quietly carrying out vital functions that keep us alive and kicking. From breaking down food for energy to regulating chemical reactions, enzymes are the ultimate multitaskers in the intricate dance of life. Without these biological catalysts, processes would grind to a halt, leaving chaos in their wake.
Think of enzymes as tiny powerhouses that kickstart reactions with precision and speed, ensuring everything runs smoothly behind the scenes. They act like master chefs in a bustling kitchen, orchestrating each step to perfection without missing a beat. Enzymes not only make things happen but also control when and how they happen – talk about being true maestros in the symphony of life.
In essence, enzymes are the backbone of all living organisms, from bacteria to humans. Their ability to accelerate biochemical reactions without being consumed in the process is nothing short of miraculous. So next time you marvel at your body’s resilience or appreciate nature’s intricacies, remember that enzymes are silently working their magic to keep everything running like clockwork.
How do enzymes break down toxins in the liver?
The liver, our body’s detox powerhouse, relies on enzymes to break down toxins effectively. Enzymes like cytochrome P450 play a crucial role in metabolizing harmful substances into less toxic compounds. These enzymes work tirelessly to neutralize and eliminate various toxins we encounter daily.
By binding to specific molecules through their active sites, enzymes facilitate chemical reactions that transform toxins into harmless byproducts. This process is essential for maintaining the body’s overall health and well-being.
Enzymes in the liver undergo complex biochemical processes that enable them to recognize and target specific toxins with precision. Through a series of intricate steps, these enzymes catalyze reactions that convert toxic substances into water-soluble compounds that can be easily excreted from the body.
The efficiency of enzyme-mediated detoxification pathways highlights the remarkable adaptability and resilience of our biological systems when faced with environmental challenges.
What happens after the reaction is complete?
After the reaction is complete, the enzyme releases the products of the reaction. These products are then free to go on and participate in other cellular processes or be used for various functions within the organism. Once the enzyme has catalyzed the reaction, it returns to its original state – ready to bind with another substrate and continue its enzymatic function.
The enzyme may undergo slight conformational changes during the reaction, but it ultimately reverts back to its active form once the substrates have been transformed into products. This ability of enzymes to repeatedly facilitate reactions without being consumed themselves is what makes them essential for life.
Enzymes play a crucial role in maintaining homeostasis within cells by regulating metabolic pathways and ensuring that chemical reactions occur at appropriate rates. Without enzymes, these reactions would proceed too slowly to sustain life as we know it.
What is the function of dehydrogenase enzymes?
Dehydrogenase enzymes play a crucial role in catalyzing the removal of hydrogen atoms from specific molecules. These enzymes are essential for various metabolic processes in the body, including energy production and the metabolism of nutrients. By facilitating oxidation-reduction reactions, dehydrogenases help convert substrates into products by transferring electrons.
One significant function of dehydrogenase enzymes is their involvement in cellular respiration, where they participate in the electron transport chain to generate ATP, the cell’s primary source of energy. Additionally, these enzymes are vital for breaking down fats and carbohydrates to release energy for cellular functions.
Different types of dehydrogenases target specific substrates based on their structure and chemical properties. For example, alcohol dehydrogenase acts on alcohols, while lactate dehydrogenase works on lactate molecules.
Dehydrogenase enzymes are indispensable players in maintaining homeostasis within cells and ensuring proper functioning of biochemical pathways.
How do enzymes contribute to muscle function?
Enzymes play a crucial role in muscle function by facilitating the breakdown of nutrients to produce energy needed for muscle contraction. Within muscle cells, enzymes like creatine kinase and lactate dehydrogenase help generate ATP, the primary energy source for muscle activity. These enzymes work together to ensure that muscles have a constant supply of energy during exercise.
In addition to energy production, enzymes also aid in the repair and regeneration of muscle tissue after strenuous physical activity. Enzymes such as proteases are involved in breaking down damaged proteins within muscles, allowing for new protein synthesis and muscular growth.
Moreover, enzymes help regulate metabolic pathways within muscle cells, ensuring efficient utilization of nutrients like glucose and fatty acids for energy production. Without these enzymatic processes, muscles would struggle to maintain optimal performance during exercise.
Enzymes are indispensable players in the intricate machinery that keeps our muscles functioning effectively during various activities – from everyday movements to intense workouts.
What is the significance of the induced fit model in enzyme-substrate interactions?
The induced fit model in enzyme-substrate interactions is a fascinating concept that sheds light on the dynamic nature of enzymes. Unlike the lock and key model, this theory suggests that enzymes can change their shape to better accommodate the substrate, like a flexible glove molding around a hand. This flexibility allows for a more precise and efficient binding between the enzyme and substrate, enhancing catalytic activity.
By adjusting its shape to perfectly fit the substrate, an enzyme can increase reaction rates and specificity. The induced fit model highlights the intricate dance between enzymes and substrates, showcasing how they work together in perfect harmony to drive biochemical reactions forward.
This adaptability ensures that only specific molecules are transformed by enzymes while others are excluded. It’s like having a custom-made key that fits only one lock perfectly without any room for error or compromise. The induced fit model underscores the precision and efficiency of enzymatic reactions in living organisms.
Conclusion
Enzymes play a crucial role in speeding up biochemical reactions in our bodies. The active site of an enzyme is where the magic happens, allowing specific interactions with substrates to occur. Through various theories like the lock-and-key and induced fit models, we can better understand how enzymes bind to substrates.
From breaking down maltose by maltase to detoxifying toxins in the liver, enzymes are essential for maintaining life processes. Catalytic groups within the active site facilitate these reactions, ensuring efficiency and specificity. Enzymes also contribute to muscle function and help regulate metabolic pathways.
The induced fit model highlights the dynamic nature of enzyme-substrate interactions, emphasizing how both molecules adjust their shapes to form a perfect fit. This flexibility ensures optimal catalytic activity and specificity.
Understanding the intricacies of active sites and enzyme functions provides insights into vital biological processes that sustain life as we know it. Embracing this knowledge allows us to appreciate the remarkable precision and efficiency of enzymes in driving essential biochemical reactions throughout our bodies.