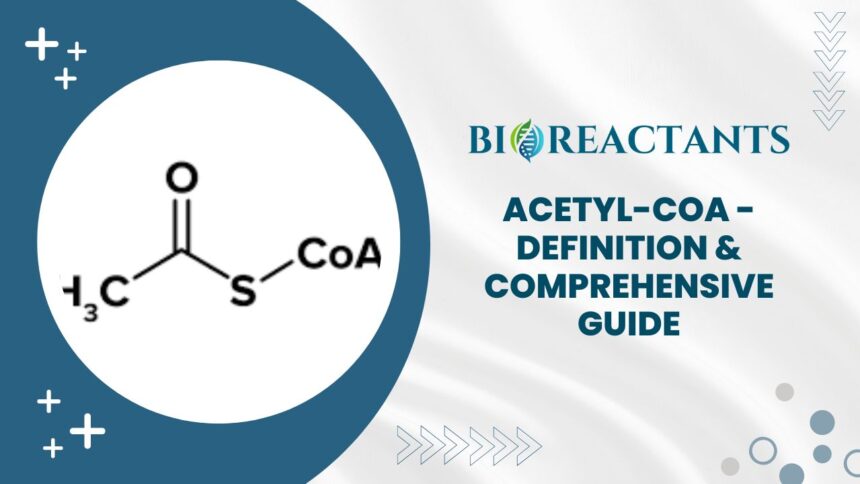
Welcome to the fascinating world of Acetyl-CoA – the powerhouse molecule that fuels cellular respiration and drives energy production in your cells! Have you ever wondered how your body converts food into usable energy? Well, Acetyl-CoA is at the heart of this intricate process. Join us on a journey to explore everything you need to know about Acetyl-CoA, from its formation to its crucial role in metabolism and beyond. Let’s dive in and unravel the mysteries of this central metabolic intermediate!
What is Acetyl-CoA, and what role does it play in cellular respiration?
Acetyl-CoA is a vital molecule that serves as a central player in the intricate dance of cellular respiration. It acts as a key intermediary metabolite, linking different metabolic pathways within the cell. When it comes to energy production, Acetyl-CoA takes center stage by fueling the Krebs cycle – a crucial step in generating ATP, the cell’s energy currency.
In simple terms, Acetyl-CoA is like the conductor orchestrating the symphony of biochemical reactions that turn nutrients into usable energy for your body. Without this molecular maestro, cells would struggle to efficiently extract and harness energy from carbohydrates, fats, and proteins.
So next time you marvel at your body’s ability to convert food into fuel, remember that Acetyl-CoA is pulling the strings behind the scenes, ensuring that every step of cellular respiration runs smoothly and effectively.
How is Acetyl-CoA formed, and where does it occur in the cell?
Acetyl-CoA, a vital molecule in cellular metabolism, is primarily formed through the breakdown of carbohydrates, fats, and proteins. When glucose undergoes glycolysis in the cytoplasm, it produces pyruvate which then enters the mitochondria to be converted into Acetyl-CoA by the enzyme pyruvate dehydrogenase complex.
Moreover, fatty acids are broken down into Acetyl-CoA through beta-oxidation in the mitochondria. Amino acids can also contribute to Acetyl-CoA production by being converted into intermediates that enter the Krebs cycle.
In terms of location within the cell, Acetyl-CoA predominantly resides in the mitochondrial matrix where it serves as a central player in various metabolic pathways such as the Krebs cycle and fatty acid synthesis. Its formation at this specific cellular site ensures efficient energy production and macromolecule synthesis essential for cellular function.
What are the primary sources of Acetyl-CoA in metabolism?
Acetyl-CoA, a crucial player in cellular metabolism, has various primary sources within the body. One significant origin of Acetyl-CoA is through the breakdown of carbohydrates. When glucose undergoes glycolysis, it forms pyruvate, which eventually gets converted into Acetyl-CoA in the mitochondria.
Fatty acids also contribute to the production of Acetyl-CoA through beta-oxidation. As fatty acids are broken down into acetyl groups, they enter the Krebs cycle as Acetyl-CoA molecules to generate energy for cellular functions.
Moreover, amino acids derived from protein metabolism can be converted into Acetyl-CoA under specific conditions. Certain amino acids like leucine and lysine can be metabolized to produce this essential molecule for energy synthesis in cells.
These primary sources provide a continuous supply of Acetyl-CoA that fuels various metabolic pathways essential for sustaining life and maintaining optimal cellular function.
Which metabolic pathways overlap with Acetyl-CoA metabolism?
Acetyl-CoA metabolism is a central player in various metabolic pathways within the cell. One prominent pathway that overlaps with Acetyl-CoA metabolism is fatty acid synthesis. Acetyl-CoA serves as the building block for fatty acid production, crucial for membrane formation and energy storage.
Furthermore, Acetyl-CoA also plays a significant role in amino acid metabolism. Through transamination reactions, Acetyl-CoA contributes to the synthesis of non-essential amino acids essential for protein production and cellular function.
Moreover, Acetyl-CoA is involved in ketogenesis, where excess acetyl units are converted into ketone bodies during periods of low carbohydrate availability. These ketone bodies can then serve as an alternative fuel source for tissues like the brain.
Additionally, Acetyl-CoA connects with cholesterol biosynthesis by contributing carbon units to form this essential lipid molecule vital for cell membranes and hormone production.
The versatility of Acetyl-CoA underscores its importance in orchestrating various metabolic processes essential for cellular homeostasis and energy balance without missing crucial details about their interconnectedness.
How does Acetyl-CoA contribute to energy synthesis in the Krebs cycle?
Acetyl-CoA, the cornerstone of cellular energy production, plays a vital role in fueling the Krebs cycle. As Acetyl-CoA enters this metabolic pathway, it combines with oxaloacetate to form citrate, kickstarting a series of reactions that ultimately generate ATP – the cell’s primary energy currency.
Through a series of enzymatic transformations within the Krebs cycle, Acetyl-CoA is broken down into carbon dioxide and high-energy electrons. These electrons are then shuttled to the electron transport chain for further energy generation through oxidative phosphorylation.
In essence, Acetyl-CoA serves as a key player in extracting stored energy from nutrients like carbohydrates and fats. By feeding into the Krebs cycle, it enables cells to produce ample ATP molecules necessary for various cellular functions ranging from muscle contraction to nerve signaling.
What happens to the acetyl carbons in the Krebs cycle?
As Acetyl-CoA enters the Krebs cycle, its two-carbon acetyl group combines with a four-carbon compound to form a six-carbon molecule. This process releases Coenzyme A and generates energy-rich molecules like NADH and FADH2.
The acetyl carbons undergo a series of reactions within the cycle, ultimately producing ATP through oxidative phosphorylation. As the cycle progresses, carbon atoms are released in the form of carbon dioxide.
These carbon dioxide molecules are exhaled when we breathe out, representing one way in which our bodies eliminate waste products from cellular metabolism. The release of these carbons is essential for maintaining equilibrium in the Krebs cycle and facilitating further energy production.
In essence, the fate of acetyl carbons in the Krebs cycle highlights the intricate dance of biochemical reactions that power our cells’ energy needs.
Is Acetyl-CoA involved in other cellular processes besides energy production?
Acetyl-CoA, the multitasking molecule in our cells, does more than just fuel our energy production. It’s like a versatile player in a cellular orchestra, participating in various processes beyond generating ATP. This superstar metabolite plays a crucial role in lipid metabolism by serving as a building block for fatty acids and cholesterol synthesis.
Moreover, Acetyl-CoA contributes to the production of important compounds such as heme, which is essential for hemoglobin formation and oxygen transport within our bodies. In addition to its involvement in metabolic pathways, this molecule also has a hand in regulating gene expression through histone acetylation.
Furthermore, Acetyl-CoA acts as a precursor for the synthesis of neurotransmitters like acetylcholine, playing an integral part in neuronal communication. Its diverse roles highlight the significance of this central metabolic intermediate beyond solely being an energy provider.
How does the abundance of Acetyl-CoA reflect the cell’s energetic state?
Acetyl-CoA, the versatile molecule at the heart of cellular energy production, serves as a key indicator of the cell’s energetic status. The abundance of Acetyl-CoA within the cell directly correlates with its metabolic activity and overall energy levels. When there is an excess of Acetyl-CoA, it signals that there is an ample supply of fuel for energy synthesis processes like the Krebs cycle.
Conversely, a depletion in Acetyl-CoA levels may suggest a shortage of available substrates needed for energy production. This dynamic relationship between Acetyl-CoA abundance and cellular energetics highlights how cells finely tune their metabolism to meet changing demands.
By closely monitoring Acetyl-CoA levels, cells can adjust their metabolic pathways accordingly to maintain homeostasis and ensure optimal energy production. This intricate balance reflects the remarkable adaptability and efficiency of biological systems in responding to varying energetic needs.
What role does Acetyl-CoA play as a second messenger?
Acetyl-CoA, a central player in cellular metabolism, also wears another hat as a second messenger. It acts as a signaling molecule that can convey information within the cell to regulate various processes. This versatile molecule has the ability to modulate gene expression and influence protein activity, impacting cellular functions beyond energy production.
Through its role as a second messenger, Acetyl-CoA can participate in intricate signaling pathways that control metabolic homeostasis and response to environmental stimuli. By serving as a molecular messenger, it connects different cellular events and coordinates responses to maintain cellular balance.
The involvement of Acetyl-CoA as a second messenger highlights its significance beyond energy synthesis. Its ability to communicate information within the cell underscores its pivotal position in orchestrating complex biological processes essential for cell survival and function.
Why is Acetyl-CoA considered a central metabolic intermediate?
Acetyl-CoA is often hailed as a central metabolic intermediate due to its versatility and significance in various cellular processes. This molecule serves as a crucial link between different metabolic pathways, playing a key role in energy production, lipid synthesis, and amino acid metabolism. Its ability to be derived from multiple sources and feed into diverse biochemical reactions highlights its importance in the overall functioning of the cell.
Moreover, Acetyl-CoA acts as a precursor for the Krebs cycle, where it undergoes oxidative decarboxylation to generate NADH and FADH2 – essential molecules for ATP production. This pivotal role in energy synthesis further solidifies its status as a central player in cellular metabolism.
Furthermore, Acetyl-CoA’s involvement in histone acetylation demonstrates its influence beyond energy generation, influencing gene expression and protein function. The interconnected nature of these pathways underscores why Acetyl-CoA is considered a linchpin molecule driving numerous cellular processes.
How do cells monitor Acetyl-CoA levels, and what modifications are dependent on it?
Cells have a sophisticated way of monitoring Acetyl-CoA levels to ensure optimal metabolic function. Enzymes like AMP-activated protein kinase (AMPK) act as sensors, detecting changes in cellular energy status. When Acetyl-CoA levels are high, it indicates an abundance of available energy for the cell’s needs.
On the flip side, low Acetyl-CoA levels signal a shortage of energy supply, prompting the cell to adjust its metabolic pathways accordingly. This dynamic regulation helps maintain cellular homeostasis and prevent imbalances that could disrupt normal physiological processes.
Modifications dependent on Acetyl-CoA include acetylation reactions that impact gene expression and protein function. By influencing these modifications, Acetyl-CoA plays a crucial role in regulating various cellular activities beyond just energy production.
The ability of cells to monitor and respond to changes in Acetyl-CoA levels underscores the intricate mechanisms at play within our bodies’ microscopic world.
What is the chemical composition of Coenzyme A (CoA)?
Coenzyme A (CoA) is a crucial molecule in cellular metabolism, composed of three main components: adenine, pantothenic acid, and cysteamine. Adenine provides the energy-carrying function, while pantothenic acid forms the core structure that links with the acetyl group. Cysteamine acts as the reactive thiol group that binds to various substrates during metabolic reactions.
The combination of these components allows CoA to act as a carrier of acyl groups in numerous biochemical pathways. This versatile molecule plays a key role in fatty acid synthesis and degradation, amino acid metabolism, and the Krebs cycle. Its ability to transfer acetyl groups makes it essential for energy production through cellular respiration.
Given its central role in multiple metabolic processes, Coenzyme A is often referred to as a “molecular lynchpin” due to its involvement at critical junctures within cellular pathways. This unique composition enables CoA to participate in diverse biochemical reactions necessary for sustaining life at the molecular level.
Which vitamin is essential for CoA production, and what are its natural sources?
Ever wonder about the vital vitamin needed for Coenzyme A (CoA) production? Look no further than pantothenic acid, also known as Vitamin B5. This essential nutrient plays a crucial role in synthesizing CoA, which is indispensable for various metabolic processes in our cells.
Pantothenic acid can be found naturally in a variety of foods like eggs, dairy products, meat, and legumes. Incorporating these foods into your diet ensures an adequate intake of Vitamin B5 to support CoA production and overall cellular function.
Whether you prefer indulging in a hearty omelet for breakfast or savoring a delicious bowl of yogurt with nuts and fruits as a snack, you’re unknowingly fueling your body with the necessary nutrients to maintain optimal CoA levels. So next time you plan your meals, remember the importance of including Vitamin B5 sources to support healthy metabolism and energy production.
How does CoA availability relate to other vitamin-producing pathways?
Coenzyme A (CoA) availability is crucial for various vitamin-producing pathways within the cell. It serves as a cofactor in numerous enzymatic reactions that are essential for synthesizing vitamins like pantothenic acid, which is a precursor to CoA itself. Without sufficient CoA levels, these pathways may be disrupted, leading to potential deficiencies in essential vitamins.
Furthermore, CoA plays a vital role in fatty acid metabolism by forming acyl-CoA compounds necessary for beta-oxidation and energy production. This connection highlights the intricate relationship between CoA availability and the efficient utilization of nutrients within the cell.
Additionally, CoA availability can impact processes such as amino acid metabolism and cholesterol synthesis since these pathways also rely on specific enzymes that require CoA as a cofactor. Therefore, maintaining optimal levels of CoA is essential for supporting overall cellular function and metabolic balance.
Can you explain the connection between Acetyl-CoA and the Krebs cycle?
Acetyl-CoA is like the VIP guest at a party – it’s essential for the Krebs cycle to kick off. Picture this: Acetyl-CoA enters the mitochondria, ready to mingle with enzymes in the Krebs cycle. As soon as it arrives, magic starts happening.
The connection between Acetyl-CoA and the Krebs cycle is fascinating. Once inside, Acetyl-CoA joins forces with Oxaloacetate to form Citrate, setting off a chain reaction of energy production. This process generates NADH and FADH2 – crucial players in producing ATP later on.
As the party continues (or should I say, as the cycle progresses), Acetyl-CoA keeps moving through various reactions. It sheds its carbons along the way, releasing energy that fuels cellular activities. With each turn of the Krebs cycle, more ATP is produced – thanks to our star player, Acetyl-CoA.
So next time you hear about Acetyl-CoA and the Krebs cycle, remember: they’re like dance partners choreographing an intricate routine that powers your cells’ everyday functions.
Conclusion
Acetyl-CoA is truly a central player in cellular metabolism, serving as a crucial link between various pathways and processes within the cell. Its formation, sources, and role in energy production highlight its significance in maintaining the cell’s energetic state. Moreover, its involvement as a second messenger further underscores its versatility beyond just energy synthesis.
Understanding the intricacies of Acetyl-CoA and Coenzyme A not only sheds light on fundamental metabolic reactions but also emphasizes the interconnectedness of cellular processes. As cells constantly monitor Acetyl-CoA levels to regulate key modifications and signaling cascades, it becomes evident that this molecule plays a pivotal role in orchestrating metabolic functions.
Delving into the world of Acetyl-CoA unveils a complex yet fascinating web of biochemical interactions that drive essential biological activities at the cellular level. By unraveling its biochemistry and physiological importance, we gain deeper insights into how our bodies efficiently utilize nutrients to sustain life processes.